絲裂黴素C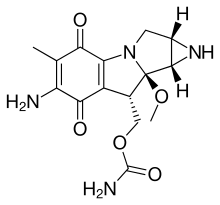 |
 |
| 臨床資料 |
|---|
| 商品名 | Mitosol, Mutamycin, Jelmyto |
|---|
| 其他名稱 | UGN-101 |
|---|
| AHFS/Drugs.com | Monograph |
|---|
| MedlinePlus | a682415 |
|---|
| 核准狀況 |
|
|---|
| 懷孕分級 | |
|---|
| 給藥途徑 | 靜脈注射、外用 |
|---|
| 藥物類別 | 抗腫瘤藥 |
|---|
| ATC碼 | |
|---|
| 法律規範狀態 |
|---|
| 法律規範 |
|
|---|
| 藥物動力學數據 |
|---|
| 藥物代謝 | 肝臟 |
|---|
| 生物半衰期 | 8–48 min |
|---|
| 識別資訊 |
|---|
{11-Amino-7-methoxy-12-methyl-10,13-dioxo-2,5-diazatetracyclo[7.4.0.02,7.04,6]trideca-1(9),11-dien-8-yl}methyl carbamate
|
| CAS號 | 50-07-7 |
|---|
| PubChem CID | |
|---|
| PubChem SID | |
|---|
| IUPHAR/BPS | |
|---|
| DrugBank | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| KEGG | |
|---|
| ChEBI | |
|---|
| ChEMBL | |
|---|
| CompTox Dashboard (EPA) | |
|---|
| ECHA InfoCard | 100.000.008  |
|---|
| 化學資訊 |
|---|
| 化學式 | C15H18N4O5 |
|---|
| 摩爾質量 | 334.33 g·mol−1 |
|---|
| 3D模型(JSmol) | |
|---|
| 熔點 | 360 °C(680 °F) |
|---|
| 水溶性 | 8.43 g L−1 |
|---|
CO[C@@]12[C@H](COC(N)=O)C3=C(C(=O)C(C)=C(N)C3=O)N1C[C@@H]1N[C@@H]12
|
InChI=1S/C15H18N4O5/c1-5-9(16)12(21)8-6(4-24-14(17)22)15(23-2)13-7(18-13)3-19(15)10(8)11(5)20/h6-7,13,18H,3-4,16H2,1-2H3,(H2,17,22)/t6-,7+,13+,15-/m1/s1  Y YKey:NWIBSHFKIJFRCO-WUDYKRTCSA-N
|
絲裂黴素C(英語:Mitomycin C)是化療藥物絲裂黴素的一種,分子式C15H18N4O5,1950年代由日本科學家在鏈黴菌屬的Streptomyces caespitosus中發現[5]。
參考文獻[編輯]
- ^ Reiss, G.J. KUWQIF: Mitomycin C Dihydrate, also known as (6-amino-8a-methoxy-5-methyl-4,7-dioxo-1,1a,2,4,7,8,8a,8b-octahydroazireno[2',3':3,4]pyrrolo[1,2-a]indol-8-yl)methyl carbamate dihydrate. Cambridge Structural Database: Access Structures (Cambridge Crystallographic Data Centre). 2011 [2021-11-03]. doi:10.5517/ccdc.csd.cc12bt29. (原始內容存檔於19 November 2021).
- ^ Mitomycin (Mutamycin) Use During Pregnancy. Drugs.com. 19 August 2019 [15 April 2020]. (原始內容存檔於22 October 2020).
- ^ Mitocin mitomycin 20 mg powder for injection vial (370360). Therapeutic Goods Administration (TGA). 12 August 2022 [29 April 2023]. (原始內容存檔於18 March 2023).
- ^ Mitocin (Echo Therapeutics Pty Ltd). Therapeutic Goods Administration (TGA). 28 September 2022 [29 April 2023]. (原始內容存檔於18 March 2023).
- ^ Tomasz M. Mitomycin C: small, fast and deadly (but very selective). Chemistry & Biology. September 1995, 2 (9): 575–579. PMID 9383461. doi:10.1016/1074-5521(95)90120-5
 .
.
外部連結[編輯]
- Clinical trial number NCT02793128 for "The OLYMPUS Study - Optimized DeLivery of Mitomycin for Primary UTUC Study (Olympus)" at ClinicalTrials.gov


 .
.
